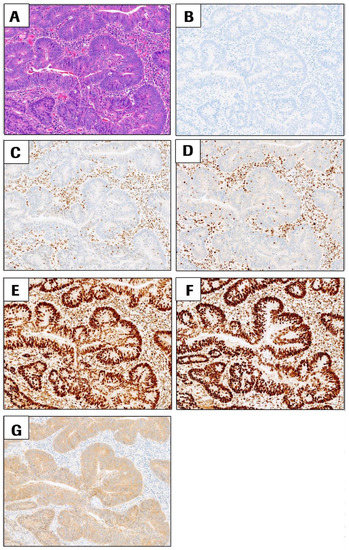
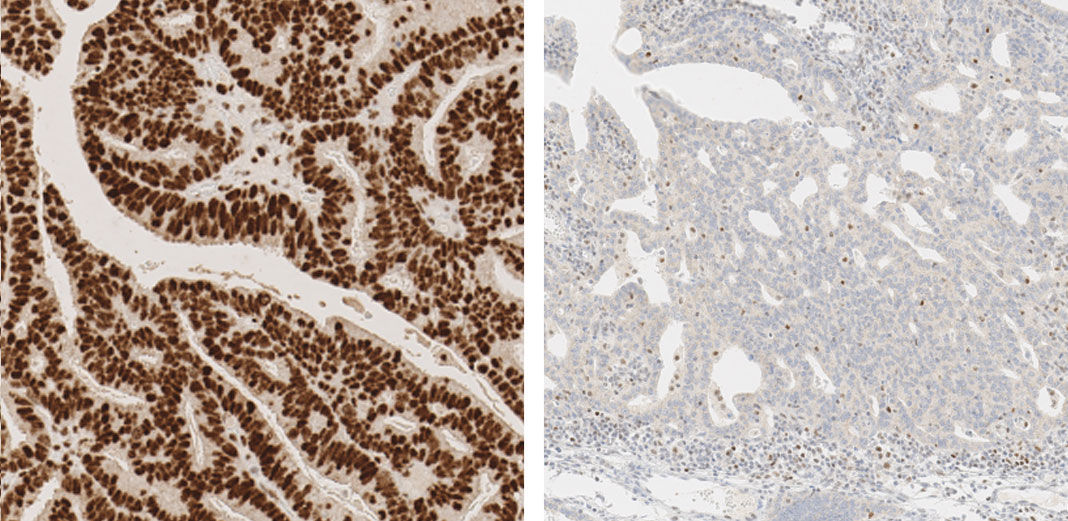
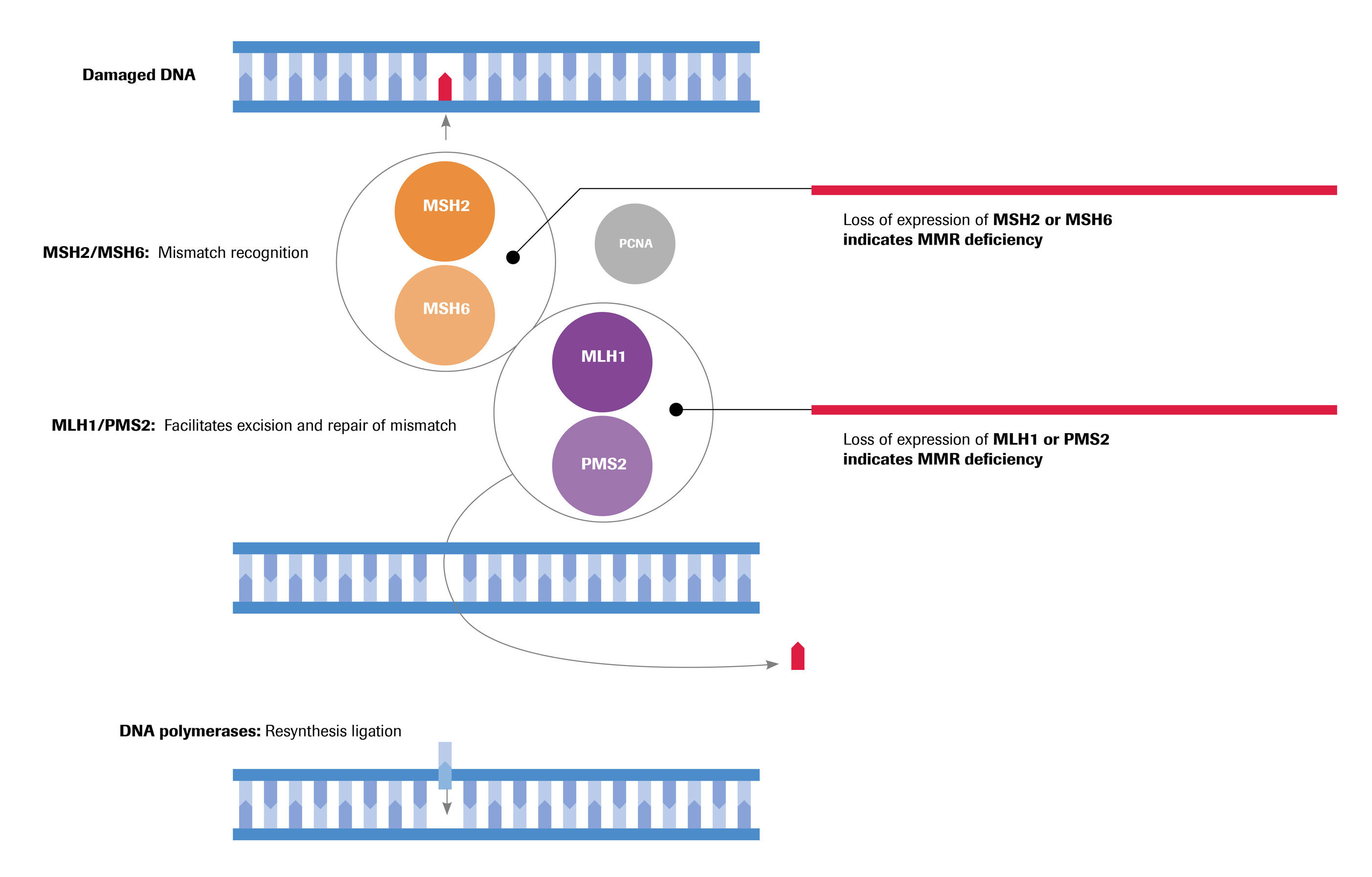
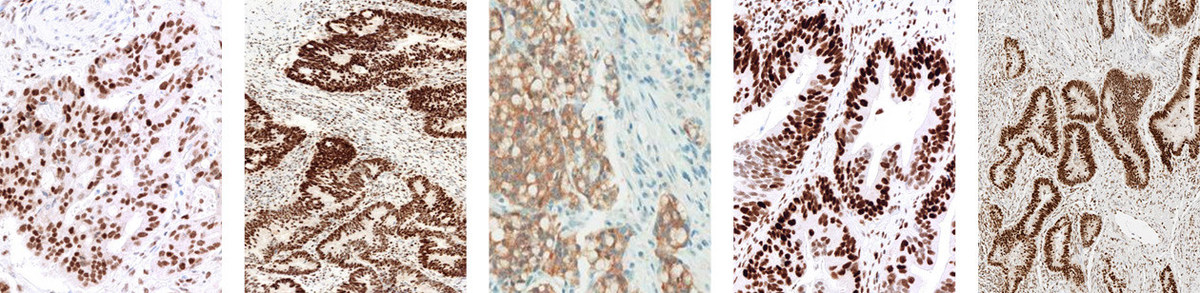
Detecting mismatch repair deficiency in solid neoplasms: immunohistochemistry, microsatellite instability, or both? - Modern Pathology

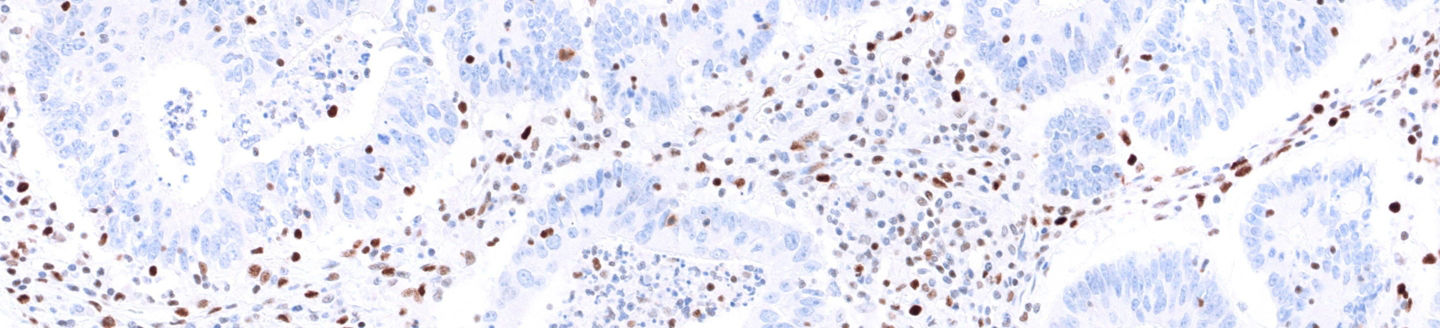
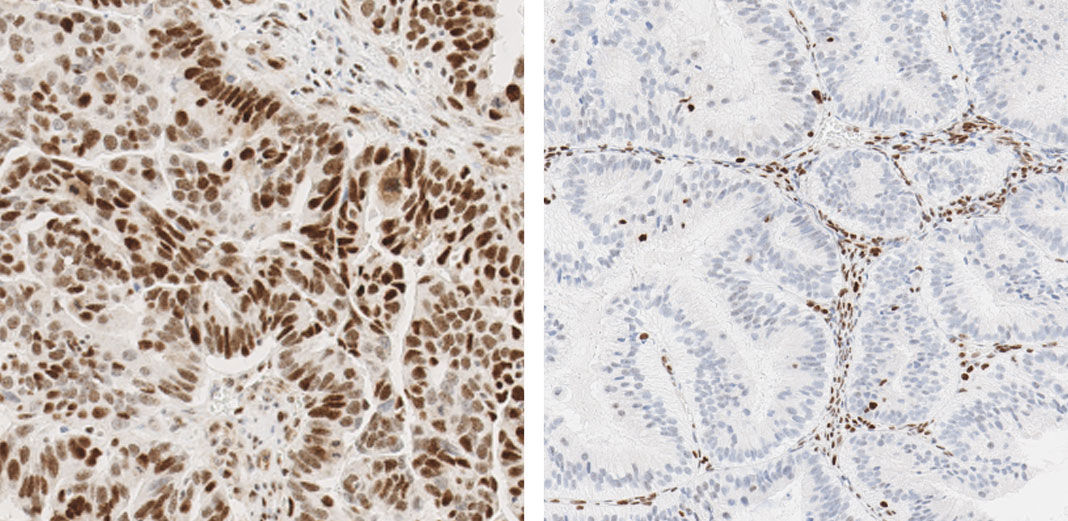
Mismatch repair markers in preoperative and operative endometrial cancer samples; expression concordance and prognostic value | British Journal of Cancer